Background: Ponatinib is a BCR::ABL1 TKI currently approved to potently inhibit all known native and single resistance mutation variants of BCR::ABL1, including T315I. The phase 2 OPTIC (Optimizing Ponatinib Treatment in CP-CML, NCT02467270; ongoing) trial is evaluating the efficacy and safety of ponatinib using a novel, response-based, dose-reduction strategy in patients with CP-CML whose disease is resistant to ≥2 TKIs or who harbor T315I. The response-based dosing strategy aims to optimize efficacy and improve the safety of ponatinib. Results from the OPTIC primary analysis and the 3-year update demonstrated an improved risk:benefit ratio for ponatinib starting with 45 mg/d, followed by dose reduction to 15 mg/d upon attaining ≤1% BCR::ABL1IS. We present for the first time the long-term 4-year update of efficacy and safety outcomes from the OPTIC trial.
Methods: Patients with CP-CML resistant to ≥2 TKIs or with the BCR::ABL1T315I mutation were randomized to starting doses of ponatinib 45 mg, 30 mg, and 15 mg once daily. Upon achievement of ≤1% BCR::ABL1IS, doses were reduced to 15 mg in the 45-mg and 30-mg cohorts. The primary endpoint was ≤1% BCR::ABL1IS at 12 months; secondary endpoints included molecular response rates and safety outcomes, including arterial occlusive event (AOE) rates that were adjudicated prospectively by an independent review committee. Probabilities of progression-free survival (PFS) and overall survival (OS) by 48 months were estimated using the Kaplan-Meier method.
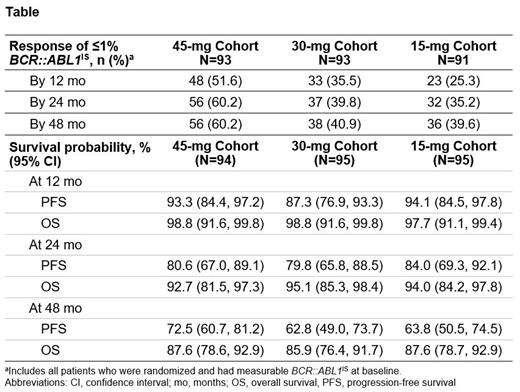
Results: A total of 283 patients were randomized (45 mg/30 mg/15 mg: n=94/95/94). The median age was 48 years (range: 18‒81 years). As of May 8, 2023, 60.2% (56/93), 40.9% (38/93), and 39.6% (36/91) of patients in the 45-mg, 30-mg, and 15-mg cohorts, respectively, achieved ≤1% BCR::ABL1IS by 48 months ( Table). Median duration of response was not reached in any cohort. Of the patients with T315I mutations, 64.0% (16/25), 25.0% (5/20), and 15.8% (3/19) achieved ≤1% BCR::ABL1IS by 48 months versus 60.0% (30/50), 46.6% (27/58), and 45.3% (24/53) of patients without mutations and 56.3% (9/16), 40.0% (6/15), and 50.0% (9/18) of patients with other mutations in the 45-mg, 30-mg, and 15-mg cohorts, respectively. Of the patients who achieved ≤1% BCR::ABL1IS, 80.4% (45/56) and 71.1% (27/38) in the 45-mg and 30-mg cohorts, respectively, had dose reductions to 15 mg upon achieving ≤1% BCR::ABL1IS;11 patients did not reduce dose upon achieving ≤1% BCR::ABL1IS, including 6 dose reductions for adverse events (AEs; 3 maintained response), 2 discontinuations for AEs, and 3 lost to follow-up/other. Of the 86.7% (13/15) and 55.6% (5/9) of patients in the 45-mg and 30-mg cohorts who had dose re-escalations after loss of ≤1% BCR::ABL1ISresponse, 69.2% (9/13) and 80.0% (4/5) regained a ≤1% BCR::ABL1ISresponse, respectively. The median time to regain response after dose re-escalation among patients who achieved ≤1% BCR::ABL1ISresponse was 126 days (95% CI, 39-167) in the 45-mg cohort and was not applicable in the 30-mg cohort. Of the patients who did not regain response, 3 discontinued due to AE or progressive disease and 1 remains on treatment. Median PFS (72.5 months) was highest in the 45-mg cohort. Most common grade ≥3 treatment-emergent AEs were thrombocytopenia (27.3%), neutropenia (17.7%), and hypertension (9.9%). Exposure-adjusted AOE rates (95% CI) were 3.87 (1.45, 6.30) in the 45-mg cohort, 3.66 (1.11, 6.20) in the 30-mg cohort, and 1.73 (0.02, 3.44) 15-mg cohort.
Conclusions: Results from the first long-term analysis of the OPTIC study support ponatinib's robust long-term efficacy and manageable safety profile in patients with highly resistant CP-CML. These results are also consistent with previous analyses of the OPTIC trial and demonstrate that a ponatinib starting dose of 45 mg/d with reduction to 15 mg/d upon attainment of ≤1% BCR::ABL1IS continued to provide the optimal benefit:risk ratio.
Disclosures
Cortes:Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Research Funding; Takeda: Consultancy, Honoraria; Gilead: Consultancy; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Forma Therapuetic: Consultancy. Deininger:Leukemia & Lymphoma Society: Research Funding; DisperSol: Research Funding; SPARC: Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; CTO BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sangamo: Consultancy, Membership on an entity's Board of Directors or advisory committees; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; DisperSol: Consultancy; Medscape: Consultancy; Fusion Pharma: Consultancy; Cogent: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: part of a study management committee, Research Funding; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: part of a study management committee, Research Funding. Lomaia:Novartis: Other: Travel, accommodation, and expenses, Speakers Bureau; Pfizer: Other: Travel, accommodation, and expenses, Speakers Bureau; Fusion Pharma: Speakers Bureau. Moiraghi:Takeda: Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Speakers Bureau. Undurraga:Pfizer: Speakers Bureau; Novartis: Speakers Bureau; Janssen: Speakers Bureau; Roche: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees. Pavlovsky:Novartis: Speakers Bureau; Pfizer: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau. Chuah:Korea Otsuka Pharmaceutical: Honoraria; Bristol Myers Squibb: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Other: Travel, Research Funding. Sacha:Bristol Myers Squibb: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Speakers Bureau; Pfizer: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Adamed: Consultancy, Honoraria. Lipton:Bristol Myers Squibb: Consultancy, Research Funding; ARIAD: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. McCloskey:BluePrint Health: Speakers Bureau; Novartis: Consultancy; Blueprint Medicines: Consultancy; Bristol-Myers Squibb/Pfizer: Consultancy, Honoraria, Speakers Bureau; BluPrint Oncology: Honoraria; Amgen: Speakers Bureau; Takeda: Speakers Bureau; Incyte: Speakers Bureau; Jazz Pharmaceuticals: Speakers Bureau; Stemline Therapeutics: Speakers Bureau. Hochhaus:Pfizer: Research Funding; Incyte: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Rousselot:Incyte: Consultancy; Takeda: Consultancy; Novartis: Consultancy; Bristol Myers Squibb: Consultancy; Pfizer: Consultancy. Rosti:Pfizer: Research Funding, Speakers Bureau; Bristol Myers Squibb: Speakers Bureau; Incyte: Speakers Bureau; Novartis: Speakers Bureau. de Lavallade:Bristol Myers Squibb: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Pfizer: Honoraria; Novartis: Honoraria. Turkina:Pfizer: Other: Travel, accommodation expenses, Speakers Bureau; Novartis: Other: Travel, accommodation expenses, Speakers Bureau; Fusion Pharma: Speakers Bureau. Talpaz:Sumitomo: Research Funding; Novartis: Research Funding; Morphosys: Research Funding; BMS: Other: Advisory Board Member; Sumitomo: Other: Advisory Board Member; BMS: Research Funding. Mauro:Novartis: Consultancy, Honoraria, Other: Travel, accommodation, and expenses, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Other: Travel, accommodation, and expenses, Research Funding; Pfizer: Consultancy, Honoraria, Other: Travel, accommodation, and expenses, Research Funding; Takeda: Consultancy, Honoraria, Other: Travel, accommodation, and expenses, Research Funding; Sun Pharma/SPARC: Research Funding. Lu:Takeda: Current Employment. Vorog:Takeda: Current Employment. Apperley:Novartis: Honoraria, Speakers Bureau; Pfizer: Research Funding; Bristol Myers Squibb: Honoraria, Speakers Bureau; Incyte: Honoraria, Research Funding, Speakers Bureau.